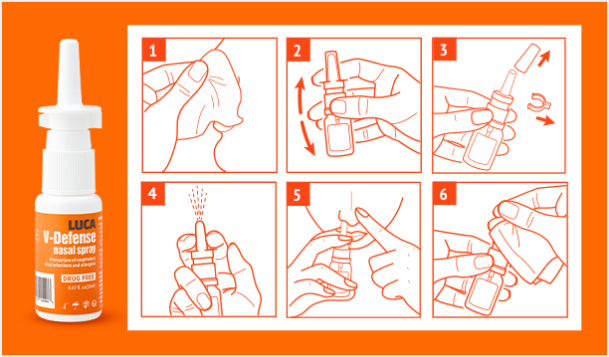
LUCA AICELL


LUCA V-Defense Nasal Spray
Prevention of respiratory viral infections
By forming a physical antimicrobial film on the nasal mucosa, it helps relieve rhinitis symptoms and
prevents respiratory viruses (COVID-19, flu, etc.) and allergens from penetrating the nasal mucosa.

Prevention of respiratory
Viral infections
- • A Protective Barrier in your Nose
- • Fast, Gentle, and Drug-Free
- • Safe for Daily Use by Adults and Kids
- • Helps relieve rhinitis symptoms
- • Allergy prevention
- • Respiratory virus prevention
- • Mask replacement
How it works
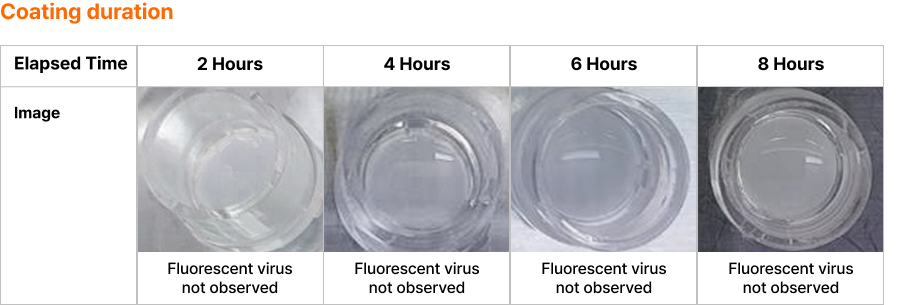
Antibacterial coating duration test result
After coating the nasal mucosal tissue with LUCA V-Defense and injecting a fluorescent virus,
as a result of the coating duration test, it was confirmed that the coating lasted up to 8 hours in a laboratory environment.
There may be individual differences in the actual situation of the nasal mucosa, but the coating lasts about 4 to 5 hours.
✓ LUCA V-Defense Coating and Fluorescent Virus Administration
- To administer the same fluorescently tagged virus-mimicking cells to artificial nasal mucosal tissue and to tissues that have not sprayed LUCA V-Defense
- Check the coating effect of LUCA V-Defense by checking how much fluorescent virus absorbed in mucosal cells after a certain period of time is present in the test group and the control group
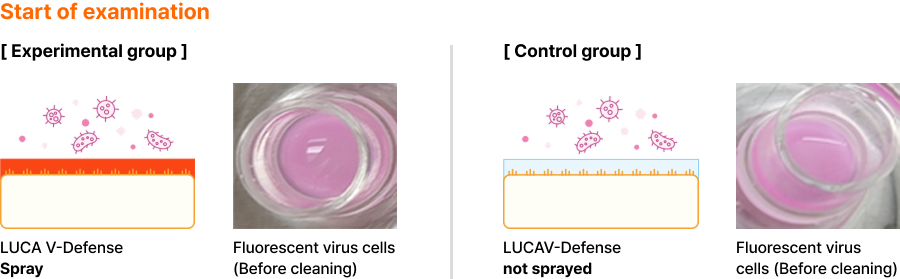
Fluorescent virus cleaning


✓ Checking LUCA V-Defense Coating Duration in Artificial Nasal Mucosal Tissue
- Check the coating duration of Luca V Defense by spraying Luca V Defense on artificial nasal mucosal tissue and fluorescent virus after a certain period of time
- Ensure coating continues to prevent virus from entering mucous membranes for up to 8 hours in a laboratory environment
Key ingredients

Lambda Carrageenan
Protect membrane
Carrageenans are a family of natural linear sulfated polysaccharides. They are extracted from red edible seaweeds. Carrageenan powered mucoadhesive formula, helping spray coat in nasal cavity to provide long-lasting moisture and sinus relief.

Span 20
Nano coating
Span 20 is a type of phospholipid that forms a phospholipid bilayer when sprayed on the nasal mucosa, thereby coating the nasal mucosa. The manufacturer, LUCA AICell, possesses nanocoating technology and is developing various applications using phospholipids.

Menthol
Easier to breathe
Menthol works as a decongestant, shrinking swollen membrane in the nose and making it easier to breathe. its cooling effect can help your nasal passages feel more open. Relieve dizziness, faint, nausea and Refresh and activate mind and body

Eucalyptol
Clearer breathing
For many years, eucalyptus oil has been used to loosen mucus. Relieve nasal congestion due to cold, headache and dizziness Boost deeper and clearer breathing to feel revitalize and energize as well as stop the clogging of mucus in nasal path.
Research/Quality Control
Papers related to efficacy


-

Nasal sprays have been shown to be a very effective way to block the transmission of the virus to humans.
Formulation of a Composite Nasal Spray Enabling Enhanced Surface Coverage and Prophylaxis of SARS-CoV-2
July 2021 Advanced Materials 33(26):2008304- • On application, nasal sprays directly contact the nasal mucosa lining the epithelium.
- • Demonstration of potential removal of the virus via trapping within the sprayed layer and elimination through natural nasal clearance mechanisms (sneezing/nose-blowing/swallowing).
- • Demonstration of potential blocking of virus uptake into the cells as the polymer creates a steric barrier across the cell interface.
- • And demonstration of potential inhibition of virus uptake by creating a steric barrier around the interface of the virus.
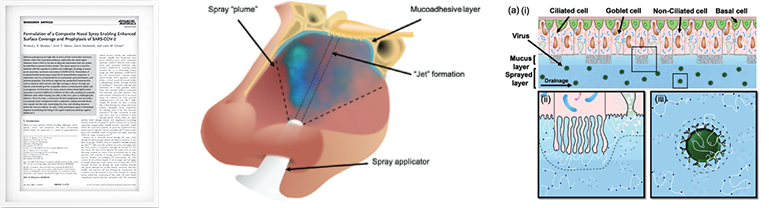
-

As a result of testing the carrageenan coating ability, it was confirmed that the multi-layer composition was perfectly coated on the surface and protected by forming a nano-sized physical film.
K-carrageenan/chitosan nanolayered coating for controlled release of a model bioactive compound
Innovative Food Science and Emerging Technologies 16 (2012) 227-232- • Carrageenan and chitosan nanolayers were demonstrated using equipment capable of measuring nanoscale, such as SEM images.
- • The SEM image of the coating with MB in the 6th layer was shown as an example.
- • The total thickness of the carrageenan/chitosan layers was 234.9 nm (Fig.4 a).
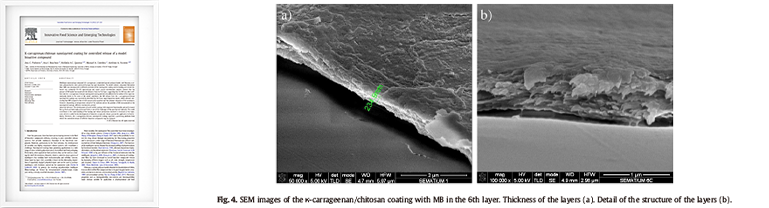
-

As a result of the carrageenan film formation experiment, excellent coating ability and film retention ability were confirmed
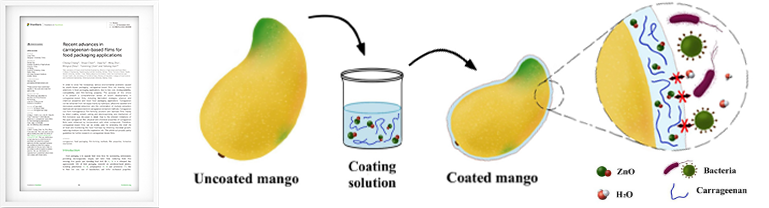
Recent advances in carrageenan-based films for food packaging applications
Frontiers in Nutrition, 10.3389 09 Sep. 2022- • The film-forming function of carrageenan and its principle were explained, and its application was demonstrated.
- • As one of the methods, carrageenan was coated on fruit (mango), and the coated fruit was fresher for 14 days than the uncoated fruit. Because the addition of zinc oxide nanoparticles allowed the composite coating to significantly increase the tensile strength by 43%, reduce the water vapor transmission by 9%, and inhibit the growth of E. coli, compared with that of pure carrageenan coatings.
-

It has been confirmed that carrageenan is a safe ingredient that is not absorbed into the human body because it has a large molecular weight and cannot be absorbed into the nasal mucosa or skin.
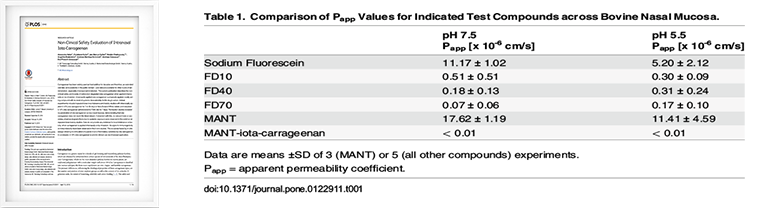
Non-Clinical Safety Evaluation of Intranasal Iota-Carrageenan
PLOS ONE | DOI:10.1371/journal.pone.0122911, April 13, 2015- • It was confirmed that carrageenan was not absorbed into Bovine Nasal Mucosa by mixing MANT dye with carrageenan.
- •MANT dye is a substance absorbed by Bovine Nasal Mucosa, but it was measured that it was not absorbed by Bovine Nasal Mucosa when mixed with carrageenan.
- •A pH of 7.5 indicates normal bovine nasal mucosa and a pH of 5.5 indicates inflamed bovine nasal mucosa.
-

A physical barrier is created, providing an extra layer of protection for the nasal cavity. Airborne allergens and pollution are prevented from reaching the sensitive lining of the nose (Allergy and PM dust)
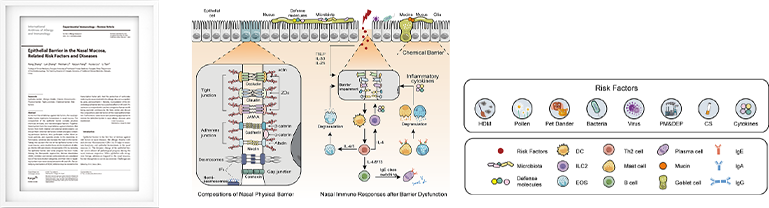
Epithelial Barrier in the Nasal Mucosa, Related Risk Factors and Diseases.
International Archives of Allergy and Immunology | DOI:10.1159/000528969, Feb 1, 2023- • It forms an additional physical barrier to the nasal mucosa.
- • It prevents allergens from sticking to the nasal mucosa and causing an allergic reaction.
- • Based on its principle, the physical layer does definitely work to alleviate allergic reaction.
- • According to the clinical results of the paper, it is effective in preventing allergic symptoms by 73%.
Test Report


-

The Intracutaneous reactivity test,
Skin sensitization test, and cytotoxicity test, all passed without problems.Intracutaneous reactivity test in Rabbits
- • As a result of conducting an intradermal reaction test on three female rabbits,
the intradermal reaction grade was 0 for all three rabbits, with no erythema, swelling, or irritation.

Skin Sensitization Test in Guinea Pig (GPMT)
- • A skin sensitization test was conducted on five guinea pigs,
LUCA V-DEFENSE NASAL SPRAY applied to this test did not show any skin reaction due to sensitization.

Cytotoxicity Test using L929 cells (Elution Test)
- • LUCA V-DEFENSE NASAL SPRAY was applied to L929 cells and the cell survival rate was checked,
and 77.51% of the cells survived. Through this experiment, it was confirmed that the product does not cause cytotoxicity.

- • As a result of conducting an intradermal reaction test on three female rabbits,
-

The stability test results, such as container extractable, shelf-life, and loss on drying test, all passed without problems.
Container Extractable Test
- • There is no adverse reaction in the results of the elution test of containers, such as property, pH, and heavy metals

Shelf-Life Test
- • All test items, including microbial testing within 3 years of validity and 6 months of opening, have been passed
Loss on Drying Test
- • Loss on drying test passed

Certifications


Korean Certifications
- • Korean Government permission has been obtained for the manufacturing of this product and for the domestic sale in Korea, and the export from Korea, of this product from the relevant Korean Government ministry, being the Ministry of Food and Drug Safety of Korea (MFDS).

Korean GMP, ISO 13485:2016
- • LUCA AICell has obtained the certification under the international standard ISO 13485:2016 and also under the domestic Korean standard, being Korea Good Manufacturing Practices of Medical Devices (GMP).

US FDA Certifications
- • This is to certify that LUCA AICell has obtained US FDA Registration & Listing Clearance on the device below.

European CE MDR certification
- • LUCA AICell has obtained European CE MDR certification.

Spanish certification
- • LUCA AICell has obtained the Spanish Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) certificate.

Malaysian MoH certification
- • LUCA AICell has obtained import and marketing authorization from the Tai FDA and the Malaysian MoH.

MoH of Indonesia and Vietnamcertification
- • LUCA AICell obtained import and sales license from MoH of Indonesia and Vietnam.

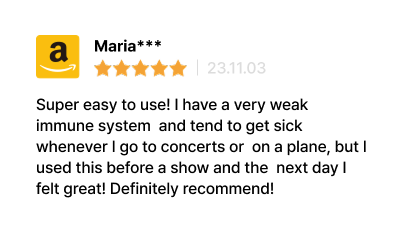
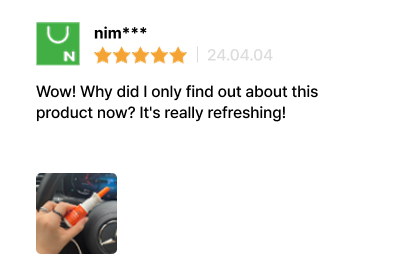
Real Customer Reviews
Review from Korea & U.S
Many customers have left reviews starting that LUCA V-Defense, which contains no steroid ingredients,
is effective in releving symptoms of rhinitis, and many recommend it for its ease of use without concerns about resistance.

This product is made by LUCA AICell,
a Korean biotech company.
LUCA AICell is a Korean biotech company developing broadspectrum antiviral therapeutics,
drug delivery systems, and diagnostic/preventive medical devices using
nanotechnology-based lipid technology.




About LUCA Contact
3rd floor, 11-35, Simin-daero
327beon-gil, Dongan-gu, Anyang-si,
Gyeonggi-do 14055, Republic of Korea
-
E-mail.
sales@lucaaicell.com
-
Tell.
031-8091-1000
-
Fax.
031-8091-0010